As Atoms make up everything that we see and touch it is not a surprise when you can see them they form together to make bonds. A chemical bond is a compound like metal, water, salt and oil. The Atom’s basically get together to make a stable structure. The different types of bonds are covalent, ionic and metallic bonds.
Plasma Spray like that from https://www.poeton.co.uk/standard-treatments/plasma-coatings is a good example of a chemical bond process. The super heated nickel in the spray is an example of a metallic bond. Metallic bonds lose electrons quickly so that their bonds tend to “float” in a sea of discarded electrons before they disappear. Alloy metals, even though they are made up of differing electrons, still have the free floaters in them.
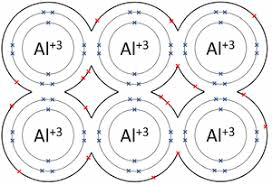
It might surprise you to find that Salt is a type of metal. It has more positively charge electrons making them ions. Therefore salt is an ionic bond.
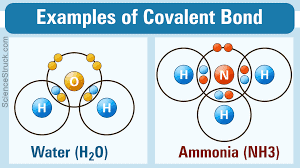
The strongest bond is that of the Covalent bond. This is where two electrons, one positive become attracted to each other and bond. One of the best examples of this is water. Water has hydrogen and oxygen working with the atom element to form into a solid state. If you add extremes of cold it will do this and freeze.

